In India, drug regulation is lax, as noted in the book The Truth Pill which deals with the ‘myth of drug regulation in India’. Adding to that is the problem of doctors not writing prescriptions legibly, although they are required to do so by law. Overlaying these is yet another issue — that of ‘look-alike, sound-alike’, or LASA, drugs. You could, especially in situations where the pharmacist is not trained, asking for one drug and getting another.
The World Health Organisation is seized of the problem. In a technical document of October 2023, the global health watchdog notes that “Look-alike, sound-alike (LASA) medicines are a well-recognised cause of medication errors that are due to orthographic (look-alike) and phonetic (sound-alike) similarities between medicines, which can be confusing. Confusions can occur between brand-brand, brand-generic or generic-generic names.”
There are a lot of LASA drugs in India. We do not know what harm they are causing because we are not even aware of them, leave alone a study. Indian drug laws require the drug regulatory body to review a trademark search to ensure that there are no misleading brand names before granting marketing authorization for a drug. However, “The very existence of countless misleading brand names shows that India’s drug regulator, Central Drugs Standard Control Organisation (CDSCO), is not doing what it is tasked to do,” says a recent scientific paper published in The Lancet Regional Health – Southeast Asia, authored by Murali Neelakantan, Parth Sharma and Ashish Kulkarni. LASA drugs may lead to significant medication errors and could quite conceivably result in harm to the patients, the paper notes, calling LASA “a significant public health threat”.
The paper gives some telling examples of drugs having same names but manufactured and marketed by different entities.
“For example, (i) brand name ‘Medzol’ is used for both Midazolam and Pantoprazole; (ii) ‘Medzole’ is used for Metronidazole oral suspension, Itraconazole capsules and Albendazole tablets; (iii) ‘Flucor’ is used for both Fluconazole and a combination of Flupentixol and Melitracen; and (iv) ‘Linamac’ is used for both Lenalidomide and Linagliptin.”
In such cases, there is no way a pharmacist could tell which drug the doctor had prescribed (in general, prescriptions in major parts of India only mention brand names with no mention of diagnosis or treatment protocol, Neelakantan et al, say.
If you check out the medindia website, you will find an astounding number of LASA drugs. Here are some examples:
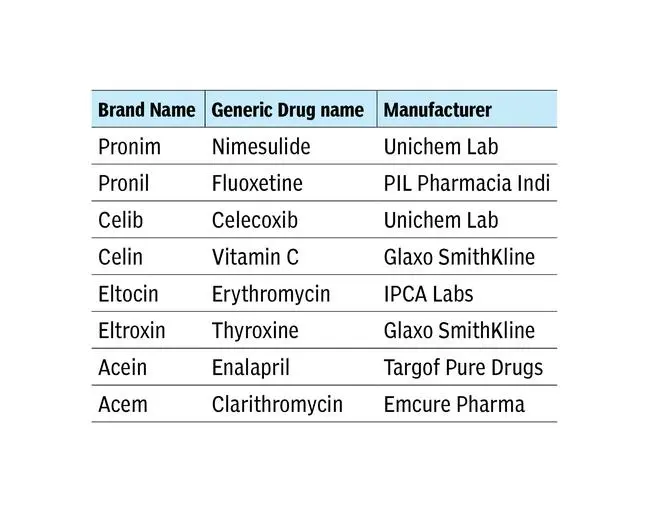
These are just some examples, but the list is big. Worse, there are LASAs produced by the same manufacturer. Examples:
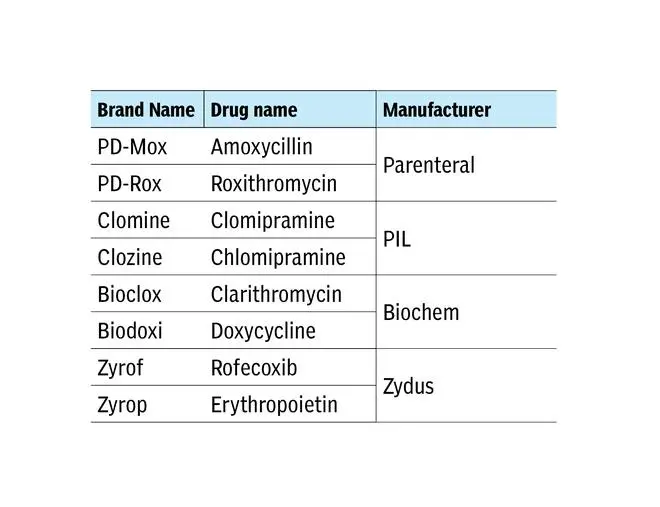
“Confusions can occur between brand-brand, brand-generic or generic-generic names. Organizations need to prospectively design and implement strategies to identify LASA medication errors and build a robust system that intercepts them before they result in patient harm,” says WHO.
“The regulator seems to have left it to pharma companies to fight each other in trademark battles to resolve the issue of misleading brand names,” the paper says.
Speaking to Quantum, Neelakantan observed that there were LASA drugs — Olvance and Oleanz for hypertension and schizophrenia respectively. Even worse is the brand name ‘Linamac’ which is used for both Lenalidomide (treating cancer) and Linagliptin (for diabetes). “Imagine what would happen if you took one for the other,” he said.
Noting that the situation could be easily remedied, Neelakantan said that for starters doctors should be mandated to also write the generic names of the drugs (like paracetamol or amoxicillin) as well as the diagnosis, and the purpose for which the drug was being prescribed (like cancer or diabetes). From the regulator’s side, authorization should be given only for unique brand names after checking that they do not sound similar or look alike another drug. He also called for ‘tall man lettering’, where the possible misleading letters are highlighted to avoid confusion (such as HydrOXYzine, an antihistamine, to distinguish it from HydALAzine, an antihypertensive).





Comments
Comments have to be in English, and in full sentences. They cannot be abusive or personal. Please abide by our community guidelines for posting your comments.
We have migrated to a new commenting platform. If you are already a registered user of TheHindu Businessline and logged in, you may continue to engage with our articles. If you do not have an account please register and login to post comments. Users can access their older comments by logging into their accounts on Vuukle.